Two-dimensional infrared (2D IR) spectroscopy opens up a deeper layer of molecular investigation, revealing vibrational couplings, structural dynamics, and intermolecular interactions at ultrafast timescales. Unlike traditional IR, which offers a static snapshot, 2D IR maps how vibrational modes interact, providing a clearer view of complex environments—essential for studying proteins, hydrogen bonding networks, and reaction dynamics.
This article lays out the fundamentals of 2D IR, serving as a guide for students stepping into the field and as a resource for experts looking to broaden their applications or collaborating with us on advanced projects. For those seeking more technical detail, hyperlinks throughout the text lead to dedicated blogs covering experimental design, pulse shaping, and data analysis.
Historical Development
If you’ve heard of 2D NMR, you’re already familiar with the concept that laid the foundation for 2D IR spectroscopy. In the 1970s, two-dimensional nuclear magnetic resonance (2D NMR) revolutionized molecular studies by analyzing the interactions between nuclear spins, providing structural and dynamic information beyond traditional one-dimensional methods. Inspired by this, scientists sought to develop a similar technique for probing molecular vibrations—leading to the birth of 2D IR.
However, the journey from concept to reality wasn’t immediate. While one-dimensional infrared (1D IR) spectroscopy has existed since the early 20th century, its limitation lies in providing static information—essentially snapshots of molecular vibrations at specific frequencies. Capturing the interactions between vibrational modes and the ultrafast dynamics of molecular systems required femtosecond (10⁻¹⁵ seconds) laser technology. This critical advancement wasn’t available until the 1990s, when advancements in ultrafast lasers and two-color pump-probe techniques laid the groundwork for 2DIR. By 2000, the first true 2D IR experiment was successfully conducted, marking a transition from theory to practice and opening new pathways for molecular research.
Fundamentals of 2D IR – How Does It Work?
At its core, 2D IR spectroscopy employs sequences of ultrafast infrared pulses to excite molecular vibrations and record their interactions. This generates a two-dimensional spectrum that reveals how vibrational modes couple and evolve over time—providing insights that traditional 1D IR cannot. This generates a two-dimensional spectrum that reveals how vibrational modes couple and evolve over time.
The Basic Process:
1.Pump Pulse Pair (Excitation):A pair of infrared pulses with controlled time and phase interval t1 excites a vibrational mode into excited states. .
2.Probe Pulse (Detection): After an additional delay t2, a third pulse probes the system, detecting the resulting vibrational state. This pulse captures how the initial excitation has evolved, creating a signal that reflects vibrational energy transfer, coupling, or relaxation.
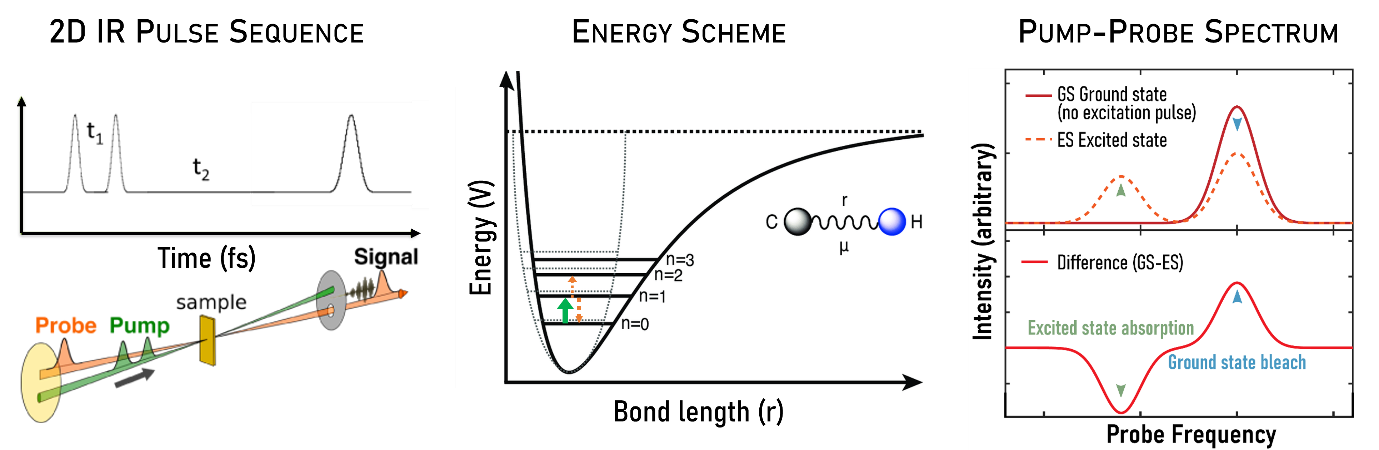
Figure 1. Principal of 2DIR
The final output—a 2D spectrum is typically presented as a 2D contour or pseudocolor plot, where the x-axis represents excitation frequency, the y-axis represents detection frequency, and signal intensity is indicated by contour lines or color gradients.
Key Features of a 2D IR Spectrum:
Diagonal Peaks:Correspond to the fundamental vibrational frequencies observed in traditional 1D IR. These represent vibrational modes without coupling.
Off-Diagonal Peaks (Cross Peaks):Indicate coupling or energy transfer between different vibrational modes. These peaks provide insight into molecular interactions and dynamic processes.
What Makes 2D IR Powerful?
Traditional IR spectroscopy, typically performed using Fourier-transform infrared (FTIR) spectrometers, measures how molecules absorb light at distinct frequencies. This is effective for identifying functional groups and providing basic structural information, but it has inherent limitations. FTIR cannot reveal how different vibrational modes within a molecule interact, nor can it capture dynamic processes such as molecular motion or changes in molecular structure over time. This restricts 1D IR to providing only a snapshot of a molecule’s static properties, missing critical insights into molecular behavior and interactions.
In contrast, 2DIR spectroscopy breaks through these limitations by introducing an additional dimension—time. Through sequential pump and probe pulses, 2D IR tracks how vibrational states influence each other and change over femtosecond timescales. This allows scientists to explore:
Coupling between vibrational modes–Off-diagonal peaks in 2D IR spectra indicate energy transfer or interactions between different modes, revealing structural dynamics.
Chemical exchange and dynamics–Real-time monitoring of hydrogen bond formation/breaking, protein folding, and ligand binding.
Environmental insights–Distinguishes between homogeneous broadening (rapid molecular motions) and inhomogeneous broadening (static environmental disorder).
The resulting 2D contour map offers significantly more detail than traditional 1D spectra. Diagonal peaks represent fundamental absorption features, while off-diagonal peaks expose hidden couplings and vibrational energy transfers—providing a much richer picture of molecular behavior.
Understanding 2D IR Spectra – Lineshape Analysis and FFCF
A key feature of 2DIR is its ability to display diagonal and off-diagonal peaks. Diagonal peaks correspond to the same absorption features seen in traditional 1D IR spectroscopy, reflecting single vibrational modes. More importantly, off-diagonal peaks indicate coupling or energy transfer between different vibrational modes. This coupling can arise when vibrational modes share the same atoms, participate in chemical interactions, or influence each other through molecular dynamics. By detecting these off-diagonal peaks, 2DIR can map the intricate connections between different parts of a molecule, providing insights into molecular structure that 1D IR cannot achieve.
In addition to revealing vibrational coupling, 2DIR offers a unique way to study dynamic behavior. The time-resolved nature of 2DIR allows scientists to track processes that occur on extremely short timescales, such as the formation and breaking of hydrogen bonds, protein folding, or rapid chemical exchanges. These dynamic processes are essential for understanding how molecules function in real-world environments, particularly in fields like biochemistry and materials science.
Another major advantage of 2DIR is its ability to distinguish between homogeneous and inhomogeneous broadening in spectral peaks. Homogeneous broadening results from rapid molecular motions and interactions within a uniform environment, while inhomogeneous broadening arises from static variations in the molecular environment. By separating these two contributions, 2DIR provides a more detailed understanding of the molecular surroundings and interactions, offering insights into both dynamic processes and the diversity of molecular environments.
-To quantify this, researchers extract the frequency-frequency correlation function (FFCF):
C(t) = ⟨δω(0) δω(t) ⟩
-Where C(t)C(t)C(t) measures how vibrational frequencies shift over time, indicating the rate at which molecular environments fluctuate.
-FFCF is often fitted to exponential decay functions, such as:
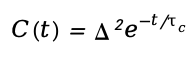
-Where Δ represents the static distribution of vibrational frequencies, and τc is the correlation time, reflecting how quickly the system relaxes.
In summary, despite the complexity of 2DIR, it provides unparalleled information, combining time resolution and frequency resolution to study molecular dynamics and static environments. This capability makes 2DIR a powerful tool for chemistry, materials science, and biology.
Examples of Applications of 2DIR Spectroscopy
2DIR’s unique ability to resolve frequencies and capture ultrafast dynamics makes it a powerful tool for studying a wide range of molecular processes. This versatility enables applications across various fields, from structural biology to materials science.
1.Molecular Structure Analysis
2DIR provides detailed insights into molecular structures by examining the coupling between different vibrational modes. 2DIR can distinguish between folding and unfolding behaviors in protein secondary structure analysis by identifying characteristic diagonal and off-diagonal peak patterns. For instance, α-helices and β-sheets exhibit distinct vibrational coupling signatures, allowing scientists to pinpoint structural changes within proteins. In addition, 2DIR is invaluable for understanding molecular coupling mechanisms. By identifying interactions within and between molecules, 2DIR supports fields like drug design, where knowing how a drug molecule binds to its target is crucial. This ability to map molecular interactions provides deeper insight into binding affinities and molecular recognition processes.
2.Chemical Exchange Dynamics
2DIR is particularly well-suited for studying fast chemical processes, such as the formation and breaking of hydrogen bonds or molecular isomerization. Its time-resolved capabilities allow researchers to track these processes in real time, offering insights that are otherwise difficult to obtain. For example, hydrogen bond dynamics can be directly observed with 2DIR. Strong hydrogen bonds, like those between phenol and toluene, typically produce a single off-diagonal peak, whereas weaker hydrogen bonds, such as those between phenol and bromobenzene, generate multiple off-diagonal peaks. By modeling these interactions, researchers can better understand solvent effects, molecular stability, and biological processes. 2DIR also detects isomerization, such as the transition between staggered and eclipsed conformations in ethane, which occurs on picosecond timescales. This capability provides a dynamic view of molecular flexibility and conformational changes.
3.Intermolecular Weak Interactions
2DIR can probe weak intermolecular interactions by tracking how vibrational frequencies shift due to environmental changes, a phenomenon known as spectral diffusion. This phenomenon captures how molecular surroundings fluctuate over time, such as through solvent rearrangement or molecular collisions. By distinguishing between inhomogeneous broadening (caused by static variations in the environment) and homogeneous broadening (caused by rapid dynamic interactions), 2DIR reveals detailed information about molecular environments. For instance, it can determine the electric field strength within a protein’s active site or the degree of solvation around a molecule. These insights are essential for understanding how molecular interactions drive chemical and biological processes.
Two-Dimensional Infrared (2DIR) Spectrum Characteristics and Analysis
Diagonal Peaks: Indicators of Molecular Vibrations
In a 2DIR spectrum, diagonal peaks reflect a molecule’s fundamental vibrational frequencies, analogous to the absorption peaks in 1D infrared spectroscopy. The shape and width of diagonal peaks provide insight into two broadening mechanisms:
Homogeneous Broadening: Arises from intrinsic molecular dynamics, such as vibrational relaxation and dephasing. This leads to narrow Lorentzian lineshapes, representing fast molecular processes occurring within the system. Homogeneous broadening is typically associated with intramolecular interactions or rapid energy exchange between the molecule and its environment.
Inhomogeneous Broadening: Results from static distributions in the molecular environment, yielding broader Gaussian lineshapes. This broadening reflects variations in the local surroundings or conformational diversity, commonly observed in solvated or heterogeneous molecular systems.
Cross Peaks: Signatures of Mode Coupling
Cross peaks are unique to 2DIR and indicate interactions or energy transfer between different vibrational modes. Their presence and intensity reveal the degree of coupling between distinct vibrational modes within the molecule (Figure 2).
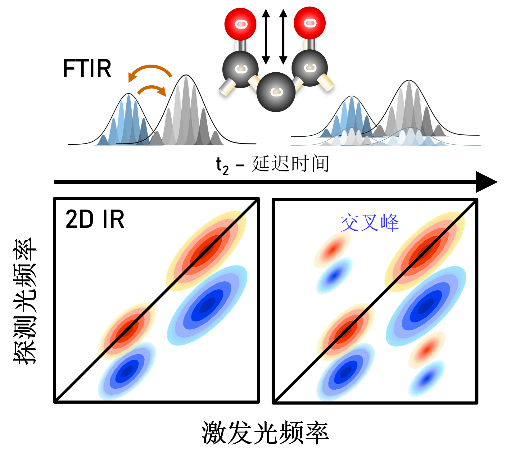
Figure 2. Schematic of Cross Peaks in Two-Dimensional Infrared (2DIR) Spectroscopy: Cross peaks appear in the 2DIR spectrum when there is an interaction between two vibrational modes. The position and intensity of these peaks reveal the degree of coupling between different vibrational modes within the molecule.
The intensity and position of cross peaks can be quantitatively described using the transition dipole coupling model:
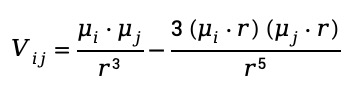
where Vij is the coupling strength between vibrational modes ii and μ represents the transition dipole moment, and is the distance between the two modes.
The term ![]() represents direct dipole-dipole coupling, inversely proportional to the cube of the distance, meaning closer vibrational modes exhibit stronger coupling.
represents direct dipole-dipole coupling, inversely proportional to the cube of the distance, meaning closer vibrational modes exhibit stronger coupling.
The directional component ![]() accounts for orientation effects, maximizing when the dipoles align along the molecular axis.
accounts for orientation effects, maximizing when the dipoles align along the molecular axis.
By analyzing cross peaks, researchers can probe vibrational coupling, solvent effects, and internal energy redistribution. For instance, in strong hydrogen-bonded systems (e.g., phenol-toluene), cross peaks appear as sharp, isolated signals, reflecting stable and well-defined interactions. In weaker hydrogen-bonded systems (e.g., phenol-bromobenzene), cross peaks may split or weaken, indicating dynamic bond formation and dissociation. 2DIR Lineshape Analysis and Frequency-Frequency Correlation Function (FFCF)
2DIR Lineshape Analysis and Frequency-Frequency Correlation Function (FFCF)
Lineshape analysis helps interpret the dynamic processes occurring within a molecular system by examining peak shapes over different delay times t2. This provides critical insight into solvation dynamics, hydrogen bonding, and vibrational energy transfer.
At short t2 times:The peak shape is primarily governed by inhomogeneous broadening, resulting in elliptical diagonal peaks. A slowly decaying FFCF indicates weak environmental fluctuations or long relaxation times.
At long t2 times: Molecular dynamics lead to peak circularization as homogeneous broadening dominates. A rapidly decaying FFCF reflects fast environmental fluctuations and short relaxation times.
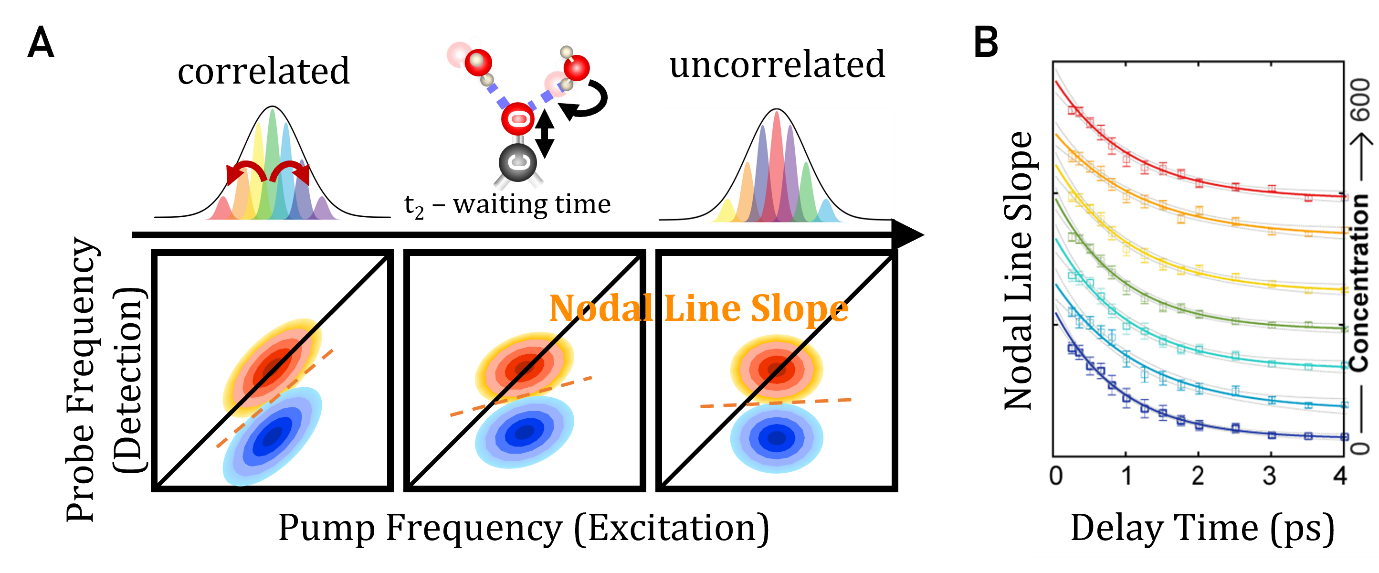
Figure 3. A. Schematic of Spectral Diffusion and Lineshape Analysis in 2DIR. Energy exchange between the vibrational probe and the surrounding solvent leads to frequency decorrelation between the pump and probe pulses.
B. The center line slope (CLS) decays exponentially as a function of t2 delay time, providing information about the local environment and relaxation dynamics.
Practical Approaches to Lineshape Analysis:
Center Line Slope (CLS) Analysis
CLS is the most widely used and direct method for tracking spectral diffusion in 2DIR spectroscopy. It measures the degree of inhomogeneous broadening by analyzing how diagonal peaks narrow or evolve with increasing t2.
CLS Calculation:
At each waiting time t2, measure the probe frequency corresponding to maximum peak absorption at each pump frequency.
Plot the peak positions and calculate the slope of this trend along the diagonal.
The CLS represents the slope of the peak’s center frequency as a function of the pump frequency.
CLS starts at 1 when the peak is fully stretched (maximum inhomogeneous broadening). CLS decreases toward 0 as the peak becomes circular (homogeneous broadening).The decay of CLS and NLS over t2 reflects the decay of the frequency-frequency correlation function (FFCF), providing direct insight into the dynamics of the environment.
Extracting the FFCF from Spectral Data
FFCF is a critical parameter describing how molecular vibrational frequencies fluctuate over time, providing a window into the dynamic processes occurring within a system. The FFCF is defined as:
C(t) = ⟨δω(0) δω(t) ⟩
where δω(t) represents the deviation of the vibrational frequency from its mean value at time t.
Exponential Decay Form of FFCF:
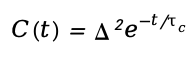
Δ represents the amplitude of static frequency distribution (inhomogeneous broadening).
τc is the correlation time, describing the timescale of environmental fluctuations.
In more complex systems, biexponential or multiexponential fits are often required to capture the contributions of multiple relaxation processes:
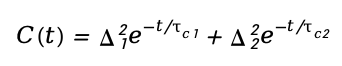
This model is essential for disentangling fast solvent relaxation from slower conformational changes.
By applying lineshape analysis techniques, 2DIR provides unparalleled insight into molecular interactions, enabling the study of dynamic processes such as hydrogen bonding, solvation, and protein folding.
Conclusion
2DIR spectroscopy offers unmatched insights into molecular structure, dynamics, and interactions. With its ability to resolve vibrational coupling, track ultrafast processes, and separate dynamic from static effects, 2DIR is a powerful tool for studying molecular systems.
In our work on complex liquid structure and dynamics, 2DIR helps us understand how molecules interact, how hydrogen bonds form and break, and how solutes behave in changing environments. These insights are crucial for advancing our knowledge of chemical and biological processes in liquids. 2DIR doesn’t just improve traditional spectroscopy — it opens new ways to explore and innovate in chemistry, biology, and materials science.



